Ozone Library
MTBE removal with ozone
In 1979, methyl tertbutyl ether (MTBE) entered the market as an oxygenate to promote cleaner combustion in gasoline. The widespread occurrence of MTBE in drinking water resources such as groundwater and surface water poses significant problems for the safety of drinking water. For example, in Santa Monica, California, several public wells had to be closed because of the occurrence of MTBE in the groundwater (1996).
Currently, there is no regulated value in place. However, the U.S. EPA has issued a consumer acceptability advisory on MTBE with a maximum tolerable concentration range in drinking water of 20-40 µg/L (on the basis of taste and odor considerations). Recently, the California Department of Health Services adopted a secondary maximum contaminant level of 5 µg/L for MTBE.
MTBE removal with ozone has become widely used from water in pump-and-treat systems and even in some drinking water systems where groundwater has high levels of MTBE. Ozone has proven to be successful at rapidly oxidizing MTBE in water. Through chemical oxidation, ozone destroys organic chemicals, breaking them down into water and carbon dioxide. For example, Kevin Wheeler of Resource Control Corp. in Rancocas, NJ, points to several cases demonstrating ozone’s effectiveness against MTBE in remediating several sites in New Jersey and Pennsylvania. At a bulk storage terminal, ozone reduced MTBE concentrations by 90% over seven months.
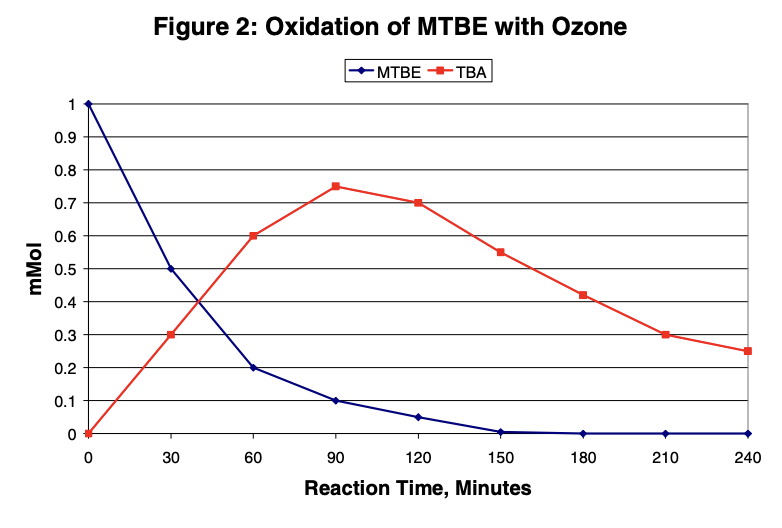
This graph shows the results for the oxidation of MTBE with 10% ozone (in oxygen). Ozone rapidly oxidizes MTBE. TBA is also oxidized but at a slower rate than MTBE.